|
Chapter 1: Physics of Light and Color |
Exploring Color Terms as an Anthropologist
Return to MODULE PAGE Return to VIDEO LAB |
by James Stanlaw (Primary Author) and Robert T. Arrigo
Some Fundamentals of Electromagnetic Radiation
Though most people cannot properly define electromagnetic radiation, everyone knows a great example of this phenomenon... light! Light is electromagnetic radiation, but so are radio waves (like those of AM/FM stereos), microwaves (useful for heating food), and X-rays (hopefully you haven't had much exposure to these!). What makes light rays so different from these other examples of electromagnetic radiation? The short answer is that we can see light, but we cannot see radio-, micro-, or X-rays.
Wavelength and Frequency
Waves of electromagnetic radiation can by quantified by numerous features, of which two, wavelength, and frequency, are particularly important. Wavelength is the measure of the separation of a wave's peaks, or valleys, and is expressed in units of feet, meters, nanometers, and so on, depending upon the magnitude of the wavelength. Frequency is a bit harder to grasp. The frequency of a wave is related to the number of peaks that pass through a given point along a wave's path in one second.
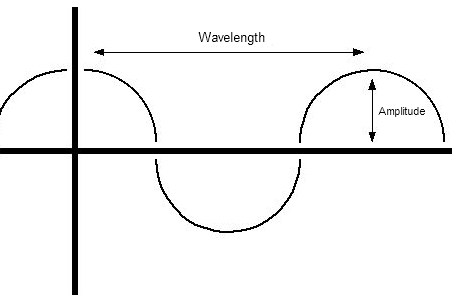
Wavelength and frequency are inversely related � as one goes up, the other goes down, and vice versa. Light is simply electromagnetic radiation within a certain range of wavelengths and frequencies that the human eye is capable of detecting. EMR waves that lie outside of these ranges are undetectable by the human eye. (To humans, the world seems dark at night, but to rattle snakes and other creatures that can see EMR of lower energy and longer wavelengths, the world is still very much visible at night.) So, there is nothing particularly special about light over other EMR phenomena, except humans can see it!
Some Fundamentals of the Human Eye
The human eye is a pretty amazing biological device. However, the eye is but the beginning of vision, not the center of it. As you may recall from a biology or anatomy class, light (electromagnetic radiation) penetrates the eye's translucent cornea, travels through the pupil and lens, and proceeds through the vitreous (the clear gelatin material that fills the eye) until it strikes special cells at the back of the eye that can detect EMR of particular wavelengths. Specifically, two types of cells, cones and rods, react to EMR of particular wavelengths. Rods are responsible for giving us (limited) night vision and are able to discern between brighter and darker light sources. Of more interest to us are cones, which are specialized cells that have three types of photosensitive proteins on their surfaces. Each type of photosensitive protein is most easily activated by EMR rays of a particular wavelength, centered around either the red, green, or blue, portion of the color spectrum. Every cone cell has proteins of each variety, but, for any given cone cell, one protein will be numerically preeminent, and thus a cone will react more to red-ish, green-ish, or blue-ish light. The more a beam of light's wavelength deviates from this concentration point, the weaker the cone's reaction will be.
Information abut the wavelengths of light detected by rods and cones as well as other visual information is sent to the rear of the brain in a special area called the primary visual cortex. It is here (among other parts of the brain) that the original signals, which merely indicated the presence of certain wavelengths of light, are processed and give rise to color perception.
And What of Color?
To find a way to describe light, we have looked at some physical properties of electromagnetic radiation and the human eye, but we haven't said much about light's most interesting aspect... color! The sensation of "seeing a color" that we experience when looking at something green, or red, or indigo, arises from the detection of EMR rays of various wavelengths. Yet, when light strikes our eye, it is generally composed of EMR rays of a great variety of wavelengths -- though red, for instance, has a wavelength of 650 nanometers, the light reflecting into our eyes off of a ripe, Red Delicious apple contains radiation of many wavelengths, not just the 650 nm type that is "ideal" red. To both describe this range of wavelengths that give rise to the perception of a color, and to allows artists and scientists to quantitatively talk about color, a system has been developed to formally describe color. Using this system, any and every color can be described by three properties: hue, saturation, and brightness.
Hue, Saturation, and Brightness
The hue of a color is that psycho-physiological sensation that we get when we see something as red, or blue, or any other color. That is, in careless, everyday parlance, hue is what "color" something is, but, for our more scientific discussion here, hue is simply one aspect of color. Specifically, a color's hue is determined by the wavelengths of light rays reaching our eyes. But, as we've discussed, the eye detects lots of different wavelengths of light, even when we are only looking at a single color. What we call hue, then, is really just the dominant wavelength of a whole slew of light waves. For instance, if we say an object is blue, it means that a majority of the light waves bouncing off of the object have wavelengths at or near 475 nm (ideal blue)... but not all.
Sometimes a color appears faded, or washed out. Take, for instance, a new pair of blue jeans. At first, the color of the jeans will be saturated with blue, but as time wears on, and the jeans are repeatedly washed, the deep blue color begins to fade from the jeans. The blue jeans becomes less saturated, and take on a grayish tone. Getting back to the scientific foundation we've established, a color's degree of saturation is related to the proportion of light rays of the dominant wavelength (e.g. 475 nm waves for blue objects) that strike the cone cells of the eye, versus all other rays present. This property of being more vivid or more faded is termed saturation. The greater the portion of light that arrive at our eyes with wavelengths of the dominant hue, the deeper, more vivid, and thus more saturated, the color.
The third color property used in this system is brightness, and it is one of the odder qualities of color. Qualitatively, brightness is the lightness or darkness of a color, and is a measure of the light's intensity. Unlike hue and saturation, however, brightness cannot be objectively measured to produce absolute values -- it can only be measured relatively. In other words, a machine (or person) can tell if one color is lighter or darker than another, but no absolute "brightness" value can be assigned. In this sense, brightness is really only a psychological construct of the mind, not a physical property of light waves.
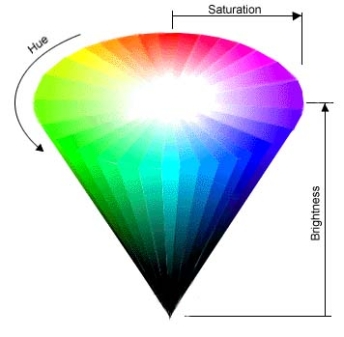 |
In this figure, we see the three properties of color in a 3D space. |
An important thing to remember, however, is that psychologically, these three variables are not independent; changing one will affect the sensation of the other two. For example, decreasing the brightness of a sample, while holding everything else constant, can alter a person's perception of the saturation. This is because the color "cone" that these properties create is lopsided and stretched out (see figures above and below), which in turn is due to the human visual apparatus not being equally sensitive in all dimensions.
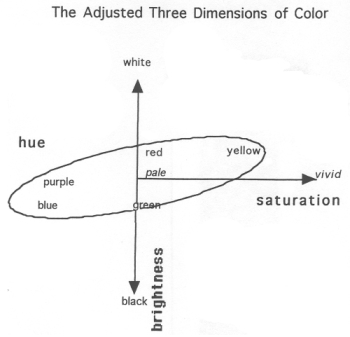 |
In this figure, we see a second representation of the three properties of color in a 3D space, but here the model has been skewed to show the uneven sensitivity of the human eye to changes in wavelength and frequecny. |