Virtual Cocaine Lab
BOOK 3: The Experiment and Lab Procedures
Paul Garris: Primary Author
Ch. 1: The Hypothesis & Overview
Introduction
This experiment will investigate how cocaine acts on dopamine neurons in the brain. Cocaine is a drug of abuse that increases extracellular dopamine levels in the nucleus accumbens by blocking the dopamine transporter. The nucleus accumbens links the limbic system (the brain network controlling motivation and emotion) to behavior. Addictive behavior is a kind of motivated behavior. Motivated behavior is divided into two components. The appetitive component consists of those behaviors related to "approaching" the goal (e.g., pressing a bar). The consummatory component represents the actual "consuming" of the goal (e.g., cocaine reaches the brain).
An important
question in the neurobiology of cocaine concerns the role of dopamine: If
cocaine increases brain dopamine when consumed, does dopamine only play a role
during the consummatory phase of cocaine use? If so, is dopamine solely related
to the reward or pleasurable aspects of cocaine consumption? Or, similar to
other motivated behaviors like sex and intracranial self-stimulation, is
dopamine involved in the appetitive phase when the rat is seeking cocaine?
Objective, hypothesis, and prediction
The overall objective of this
experiment is to investigate the role of dopamine neurons in cocaine use. Three
important questions will be asked:
(1) What happens
to brain dopamine levels when cocaine is used?
(2) When do
changes in brain dopamine levels occur when cocaine is used?
(3) How do
changes in brain dopamine levels affect behavior?
With most
research, it is often best to focus on one or two questions at a time by
simplifying the experiment. Therefore, in this experiment, we shall investigate
what cocaine does to dopamine neurons during the appetitive phase of motivated
behavior and what this action of dopamine neurons means for behavior. Thus, we
will be examining how cocaine affects the behaviors of the animal directed
towards obtaining cocaine. These behaviors have collectively been called 'cocaine
seeking'. Since we shall be monitoring
cocaine levels continuously, we should be able to observe what happens to
dopamine levels when cocaine reaches the brain.
The hypothesis
to be tested by our experiment is the following:
Hypothesis: Dopamine is involved in cocaine seeking.
To test our hypothesis, we will
make a prediction regarding the outcome of our experiments. If the outcome of
the experiment agrees with our prediction, then our hypothesis is supported.
The prediction we shall make is this:
Prediction: Dopamine release will increase during cocaine
seeking.
If our prediction is verified,
then there are
two other questions our experiment can answer:
(1) When during cocaine seeking is dopamine release increased?
(2) Does the
increase in dopamine release cause a subsequent change in behavior?
Technical issues
Determining whether dopamine is
involved in cocaine seeking requires us to consider several technical issues.
The first is that we need a laboratory animal to engage in cocaine seeking.
This issue is not too difficult, because suitably trained rats will
self-administer for cocaine.
Perhaps the
most challenging technical issue is the actual monitoring of dopamine during
cocaine seeking. Several measurement requirements must be considered: temporal
resolution (How fast is the measurement?),
selectivity (What is being
measured?), and spatial resolution
(Over what area of the brain is the measurement collected?). As described
below, the chemical microsensor technique that will be used in our experiment, fast-scan
cyclic voltammetry at a carbon-fiber microelectrode, has suitable measurement characteristics for monitoring dopamine
during cocaine seeking. .
First, the
monitoring of dopamine must be sufficiently fast to capture changes during the
cocaine seeking portion of cocaine self-administration. Rats self-administer
cocaine at a rate of about once every 10 min on average. (NOTE: Our virtual
rats will lever press more frequently.) At
this rate, even slow monitoring techniques could assess dopamine changes before
and after a lever press for cocaine. However, the goal of this experiment is to
monitor dopamine during the
behavior(s) just prior to a lever press for cocaine self-administration. During
this time, dopamine neurons may be responding to external and internal cues to
direct the rat to move towards the lever and to press it. Thus, a fast
measurement technique will be necessary to monitor dopamine levels during the
time just before the lever press. Fortunately, fast-scan cyclic voltammetry,
which monitors dopamine several times a second, is well suited for these
measurements. Therefore, voltammetry has the appropriate temporal resolution to
capture changes in brain dopamine levels during cocaine seeking.
Second, there
are many chemicals in the brain that, like dopamine, are electroactive (which means they can be measured
electrochemically). These electroactive substances could potentially interfere
with the measurement of dopamine using voltammetry and the chemical
microsensor. Fortunately, fast-scan cyclic voltammetry is a unique type of
voltammetry that collects a "chemical signature" (called a voltammogram) to identify the chemical being measured by the
microsensor. Thus, the measurement of dopamine in the rat brain by fast-scan
cyclic voltammetry is selective. However, in the present experiment, the
substance measured during cocaine seeking will be chemically compared to dopamine
that is released by the direct activation of dopamine neurons with electrical
pulses applied to a stimulating electrode implanted in the ventral tegmental
area (see Figure 1). Decades of
research have established that this type of electrical stimulation elicits
"authentic dopamine" in the rat brain. Moreover, for direct comparison, the
chemical signature for this authentic dopamine will be collected by the same
microsensor in the same area of the brain as the chemical signature recorded
during cocaine seeking behavior.
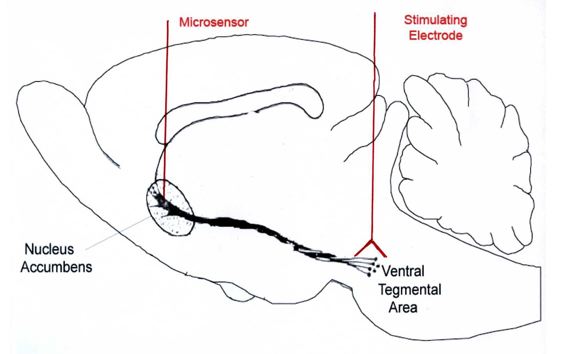
Fig. 1
Finally, it is
necessary to consider where in the brain dopamine will be monitored and the
extent of damage the implanted microsensor will cause. While several regions
could potentially be sampled, for three main reasons, the best place to record
dopamine is the nucleus accumbens.
First, this region is part of one of the major midbrain dopamine systems, the
mesolimbic dopamine system, which originates in the ventral tegmental area and
terminates in the nucleus accumbens. Second, this region links reward-related
information processed in the limbic system and cortex with motor output. Thus,
the nucleus accumbens occupies a key role in the brain circuitry supporting
motivated behavior. Third, it is well established that cocaine
self-administration will increase dopamine release in the nucleus accumbens as
measured by microdialysis. However, the probe associated with this technique is
rather large compared to the size of the nucleus accumbens. And the
microdialysis probe is known to cause damage to the adjacent tissue.
Fortunately, the carbon-fiber microelectrode used with fast-scan cyclic
voltammetry is considerably smaller. Hence, its tip can be readily implanted
within the nucleus accumbens to measure dopamine only in this region. Also,
because of smaller size, the carbon-fiber microelectrode causes considerably
less tissue damage when implanted.
Experimental design
The experimental design will be
divided into three parts:
PART 1:
Training a rat to self-administer cocaine.
In this
experiment, any animal you will work with has already been trained to
self-administer for cocaine.
PART 2:
Preparation and surgery for voltammetry measurements of dopamine.
In preparation
for brain surgery, a rat must be selected, weighed, and anesthetized
properly. You will complete all
the steps leading up to surgery. Professor Neuro will complete the surgery
procedure while you observe. When surgery is completed, the rat will have the
hardware implanted that will allow the recording of dopamine levels and the
administration of cocaine.
PART 3:
Monitoring dopamine during cocaine seeking.
Two weeks after
surgery, the rat is ready to take part in the experiment. You will perform the
experiment on a rat that has already recovered. Just before the rat is allowed
to engage in cocaine seeking, a microdrive armed with a microsensor will be
loaded into the surgically implanted hub. The microsensor will then be lowered
into the nucleus accumbens. The electrically evoked dopamine signal will be transmitted
to the computer by a wireless piece of
equipment in a backpack using radio waves or telemetry. In this way, dopamine can be monitored in the
brain of the animal without being connected or "hardwired" to the equipment.
The computer will register a lever press and send a signal to the wireless
backpack to administer cocaine.
|
|
The
wireless telemetry and drug delivery systems used in this "virtual" lab were not used in the actual experiments on which this
one was based. However, both systems are being developed and employed now.
Hence, this "virtual" lab represents some of the latest technology using
voltammetry and chemical microelectrodes to measure dopamine during cocaine
self-administration in rats. |
Completing the experiment will
require that you perform specific tasks in each of the lab areas and record
your observations or responses in your lab book.
Ch. 2: Prep area lab procedures
1)
Put on non-sterile surgical gloves.
2)
Select a rat.
3)
Weigh rat and then place the rat in the prep tray.
4)
Determine how much anesthesia to draw.
a)
per weight dose, e.g., for a pre-mixed cocktail of ketamine
and xylazine, a common anesthetic of laboratory rodents, 1 ml/kg ( inject 0.4
ml into a 400 g rat)
b)
too much anesthesia, rat dies
c)
too little anesthesia, need to inject again, but secondary
doses are tricky and rats may also die even with normal amount
5)
Grab a vile of anesthesia and a syringe.
a)
anesthesia is premixed
b)
place both objects in the prep tray
6)
Inject the needle into the vile and draw the correct amount of
anesthesia.
7)
Properly inject the anesthesia.
a)
inject into peritoneal cavity
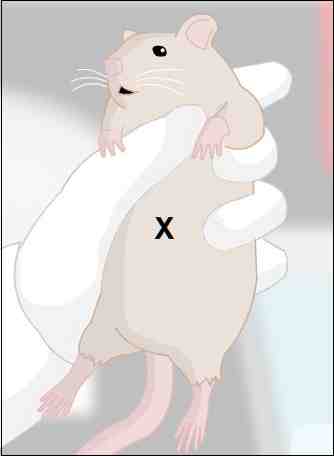
Fig. 2: Inject
the needle at the center of the 'X'.
(1)
if too high (in thoracic cavity), causes pneumothorax,
deflating lungs and killing animal
(2)
if too low (in bladder), causes complications and perhaps
death
b)
properly dispose of needle in sharps container
8)
Place the rat in the prep tray.
a)
rat needs to be on a heating pad as anesthesia prevents
thermoregulation
(1)
rat can die from being too hot or too cold
Ch.
3: Experiment area lab procedures
If this experiment were to be
completed in real time instead of through the virtual world, one would have to
perform certain tasks before beginning the actual experiment. As the
experimenter, you would be responsible for verifying that the hardware is attached
properly, secure, and that the animal is healthy. If this is not done, hardware
failure will prevent dopamine measurement or cocaine injection. If a rat is
unhealthy, it could die from cocaine injection, and the data would be skewed.
A recovered rat
will be placed in the experiment area and the telemetry unit will be attached
to the animal. The jugular injection line is flushed with saline to verify that
the line is not clogged. The microdrive is then loaded with the dopamine
microsensor to verify the microsensor functions correctly. The protective cap
covering the brain is removed from the cemented hub and the microdrive is then
attached to the hub, which was implanted during surgery into the rat's brain.
The connections are then attached between the telemetry unit and the reference
electrode (which supports the electrochemical measurements of dopamine), the
stimulating electrode, the microsensor (carbon-fiber microelectrode), and the
jugular cannula. So as not to break the microsensor, the microsensor is lowered
at the appropriate speed to the correct depth in the nucleus accumbens – the
depth that provides measurement of dopamine.
The rat must
now be allowed to habituate to the experiment area in order to feel comfortable
with its surroundings and not be stressed. A stressed animal may behave
erratically and have an adverse reaction to cocaine. The microsensors must then
be allowed to come to equilibrium in the brain so the microsensors will not
perform erratically. A test stimulation will be performed to check the
functionality of the microsensor and have it replaced if necessary, and verify
the microsensor has been placed in a dopamine-rich area so that appropriate
readings will take place.
Next, the
stimulating electrodes must be checked to make sure there is not a bad
connection, a bad stimulation unit, or the stimulating electrode has been
moved. Wireless communication must also be checked to verify there is no
problem with the battery, the telemetry pack, or the computer.
The next step
is to turn on the video camera in order to record the rat's behavior. It is now
time to begin the experiment and to monitor dopamine during cocaine
self-stimulation. The following steps will take place during the lab
experiment.
a) start
session by pressing the on button
b) session
starts with extension of lever and display of data on monitor
c) every
bar press results in
i) six
second injection of cocaine
ii) illuminating
the light above lever for 20 seconds
iii) auditory
tone for 20 seconds
iv) during
this 20 seconds, additional lever presses will not result in additional
injections of cocaine
d) session ends
i) lever
is retracted
ii) typical
session duration is 120 min (NOTE: Our's will be much, much shorter.)
e) repeat
test stimulation
i) check for functioning
microsensor
Once you have completed the
experiment, it is time for you to write up your analysis in your lab report.



